Breathing air for medical use does not meet the same criteria as industrial breathing air. Gases for medical use, including medicinal air, are regulated by the European Pharmacopoeia.
The latter is directly enforceable in France since 01/10/97 (decree of 7/11/96) and replaces the French pharmacopoeia of which only a few monographs in force remain.
The standard NFS 90-140, (air for medical use - Acceptable impurity levels .....) which served as a reference for controls until 1998 is also replaced by the European Pharmacopoeia.
The admissible pollutant contents differ, as shown in the table below:
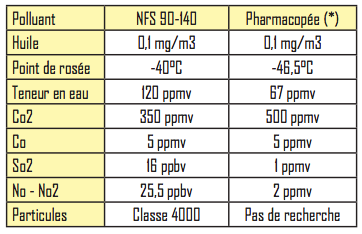
The moisture content is essential in order to avoid any risk of condensation and bacterial development. The value indicated is 1 atm, i.e. a pressure dew point of -30 ° C at around 7 bars.
It should be noted that the European Pharmacopoeia does not set any requirements in terms of particles or bacteria.

Medicinal air is a non-status product, it is not considered medical gas or medicinal gas when produced on site (Ambient air compression station + treatment) and is therefore not subject to authorization of marketing (AMM).
Our BA Series treatment plants (opposite) perfectly meet the admissible levels of pollutants required by the European Pharmacopoeia. Do not hesitate to consult our specialists for any further information on this subject.
(*) European Pharmacopoeia 2008 monograph 1238.


